Abstract
Background: Mosunetuzumab (Mosun) is a CD20xCD3 T-cell engaging bispecific monoclonal antibody (Bi-mAb) that redirects T cells to eliminate malignant B cells. Mosun is the first Bi-mAb approved for the treatment of patients (pts) with relapsed/refractory (R/R) follicular lymphoma (FL; EMA 2022) and is a fixed-duration treatment that can be administered in an outpatient setting. In a Phase II study (NCT02500407), Mosun demonstrated a high rate of complete response (CR) with a manageable safety profile in pts with R/R FL who had received ≥2 prior therapies (Budde et al. Lancet Oncol 2022). Here, we present updated data for this cohort after a median follow-up of 27 months.
Methods: Pts with FL grade (gr) 1-3a, who had received ≥2 prior therapies (including an anti-CD20 antibody and an alkylator) were enrolled. Intravenous Mosun was administered in 21-day cycles with step-up dosing in Cycle (C) 1 (Day [D] 1, 1mg; C1D8, 2mg; C1D15/C2D1, 60mg; C3D1 and onwards, 30mg). Pts achieving a CR by C8 completed treatment without additional cycles; those with a partial response or stable disease received a further nine cycles (17 total). Hospitalization following infusion was not required. The primary endpoint was CR rate determined by an Independent Review Committee. Post-hoc analyses were performed to compare efficacy outcomes with Mosun vs last prior therapy, and assess the correlation between cytokine release syndrome (CRS) and tumor response.
Results: Ninety pts with R/R FL and ≥2 prior therapies were enrolled. Median age was 60 years (range: 29-90), and 77% of pts had stage III/IV disease. Median number of prior lines of therapy was three (range: 2-10); 53% of pts were double refractory to prior anti-CD20 therapy and alkylator therapy; and 52% of pts had progressive disease within 24 months from the start of their first-line therapy. As of May 20, 2022, median time on study was 26.7 months (range: 2.0-36.2); 54 pts (60%) had completed initial treatment and 36 pts (40%) had discontinued initial treatment (25 pts [28%] due to progressive disease). Two pts (2%) were undergoing retreatment, 72 pts (80%) were in follow-up, and 16 pts (18%) had discontinued the study.
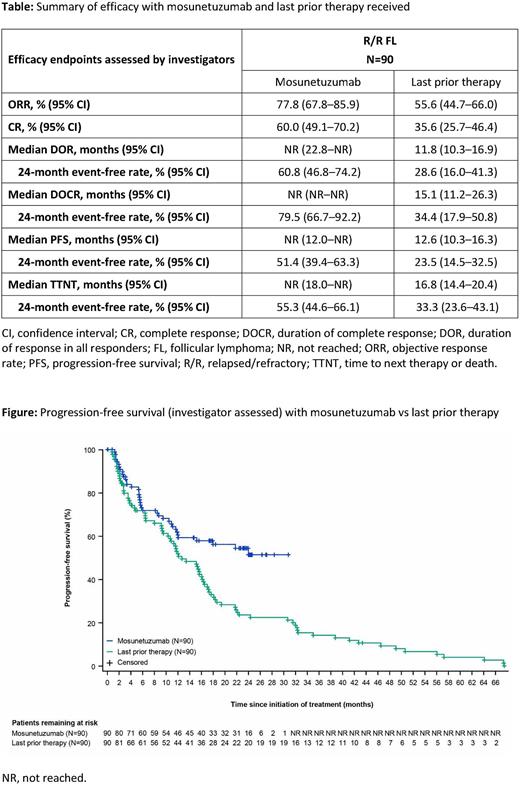
In all 90 pts, investigator (INV)-assessed objective response rate (ORR) and CR rate were 77.8% (95% CI: 67.8-85.9) and 60.0% (95% CI: 49.1-70.2), respectively. Median duration of response (DOR) and duration of CR (DOCR) were not reached (NR); 79.5% of complete responders remained in remission for at least 24 months based on Kaplan-Meier estimates. Median progression-free survival (PFS) per INV assessment was NR; 24-month PFS was 51.4% (95% CI: 39.4-63.3). The efficacy of Mosun was compared with that of pts' last prior therapy: most pts (63%) received chemoimmunotherapy as their last prior therapy, and the remainder received PI3K inhibitor-containing regimens (8%), anti-CD20 antibodies plus lenalidomide (2%), CAR-T therapy (2%), or other therapies. Response rates, PFS, DOR, DOCR and time to next therapy or death were all improved with Mosun compared with last prior therapy (Table;Figure).
No new CRS events, or fatal, serious, or gr ≥3 adverse events (AEs) were reported since the previous analysis, and no evidence of chronic toxicity was observed. Overall, the rate of AEs leading to discontinuation was low (4.4%) and no treatment-related gr 5 AEs were observed. CRS events (44.4% of pts) were mostly confined to C1 (84.5% of events) and 97.2% were gr 1/2 in severity; all CRS events resolved. No correlation was observed between the occurrence of CRS and tumor response. ORR was 77.5% and 78.0%, respectively, in pts with or without CRS events.
Conclusions: In this updated analysis, with a median follow-up of 27 months, durable responses continued to be observed with Mosun in pts with R/R FL. Compared with pts' last prior therapy, Mosun demonstrated higher ORR and CR rates, with longer DOR, DOCR, PFS and time to next therapy, although limitations should be noted for retrospective comparisons and the absence of standardized imaging assessment for the last prior therapy. The safety profile, characterized by a low rate of AEs leading to treatment discontinuation and predominantly low-grade CRS events, was consistent with previous reports and supports the administration of Mosun as an outpatient regimen. Clinical response was observed regardless of occurrence of CRS, suggesting the Mosun dose and schedule used is effective at dissociating cytokine toxicity from treatment efficacy.
Disclosures
Bartlett:Merck: Research Funding; Kite Pharma: Research Funding; Janssen: Research Funding; Forty Seven: Research Funding; Celgene: Research Funding; Bristol-Myers Squibb: Research Funding; Autolos: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche/Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Washington University School of Medicine: Current Employment; Millennium: Research Funding; Pharmacyclics: Research Funding. Sehn:Chugai: Consultancy, Honoraria; Teva, Roche/Genentech: Consultancy, Honoraria, Research Funding; AbbVie, Acerta, Amgen, Apobiologix, AstraZeneca, BMS/Celgene, Debiopharm, Genmab, Gilead, Incyte, Janssen, Kite, Karyopharm, Lundbeck, Merck, Morphosys, Novartis, Sandoz, Seattle Genetics, Servier, Takeda, TG Therapeutics, Verastem: Consultancy; AbbVie, Acerta, Amgen, Apobiologix, AstraZeneca, BMS/Celgene, Gilead, Incyte, Janssen, Kite, Karyopharm, Lundbeck, Merck, Morphosys, Sandoz, Seattle Genetics, Servier, Takeda, TG Therapeutics, Verastem: Honoraria. Matasar:Rocket Medical: Consultancy, Research Funding; Teva: Consultancy; Takeda: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; AstraZeneca: Consultancy; ADC Therapeutics: Consultancy, Honoraria; Janssen: Honoraria, Research Funding; Pharmacyclics: Honoraria, Research Funding; ImmunoVaccine Technologies: Honoraria, Research Funding; GlaxoSmithKline: Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Current equity holder in private company; IGM Biosciences: Research Funding; TG Therapeutics: Consultancy; Karyopharm: Consultancy; IMV Therapeutics: Consultancy, Honoraria; Juno Therapeutics: Consultancy; Genentech, Inc.: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche Ltd.: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy; Bayer: Consultancy, Honoraria, Research Funding. Schuster:AbbVie: Research Funding; Adaptive Biotechnologies: Research Funding; AstraZeneca: Consultancy; BeiGene: Consultancy; Celgene: Consultancy, Honoraria, Research Funding; DTRM: Research Funding; Fate Therapeutics: Consultancy; Genentech: Consultancy, Research Funding; Genmab: Consultancy; Incyte: Consultancy, Research Funding; Juno Therapeutics: Consultancy, Research Funding; Legend Biotech: Consultancy; Loxo Oncology: Consultancy; Merck: Research Funding; MorphoSys: Consultancy; Mustang Biotech: Consultancy; Nordic Nanovector: Consultancy; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding; Pharmacyclics: Research Funding; Regeneron: Consultancy; Roche: Consultancy, Research Funding; TG Therapeutic: Research Funding. Assouline:Genentech/Roche, Astra Zeneca, Novartis, BMS, Jazz, Gilead, Amgen, Beigene, Abbvie, Paladin: Consultancy, Honoraria; Novartis: Research Funding. Kuruvilla:Medison Ventures: Consultancy; Abbvie: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria, Other: DSMB; Merck: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Astra Zeneca: Honoraria, Research Funding; Amgen: Honoraria; Janssen: Honoraria; Novartis: Honoraria; Antengene: Consultancy; Seattle Genetics: Consultancy, Honoraria; Inctye: Honoraria; Pfizer: Honoraria; Lymphoma Canada: Membership on an entity's Board of Directors or advisory committees. Canales:Karyopharm: Consultancy; Janssen: Consultancy, Speakers Bureau; Incyte: Consultancy; Kite: Consultancy, Speakers Bureau; BMS: Consultancy; Beigene: Consultancy; La Paz University Hospital: Current Employment; Kyowa: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Sanofi: Consultancy; Takeda: Consultancy, Speakers Bureau; Sandoz: Speakers Bureau; Amgen: Speakers Bureau. Dietrich:Roche: Consultancy; Gilead: Consultancy; Kite: Consultancy; University Hospital Heidelberg: Current Employment. Ku:Antengene: Consultancy; Genor BioPharma: Consultancy; Roche: Consultancy; St Vincent's Hospital: Current Employment. Nastoupil:Genentech/Roche, MEI, Takeda: Other: DSMC; ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Genmab, Janssen, MEI, Morphosys, Novartis, Takeda: Honoraria; BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Genmab, Janssen, IGM Biosciences, Novartis, Takeda: Research Funding. Wei:F. Hoffmann La Roche, Ltd.: Current equity holder in private company, Current holder of stock options in a privately-held company, Patents & Royalties; Genentech, Inc.: Current Employment. Yin:F. Hoffmann La Roche, Ltd.: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months; Genentech, Inc.: Current Employment, Patents & Royalties. To:Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Huang:F. Hoffmann La Roche, Ltd: Current Employment. Penuel:Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company.
OffLabel Disclosure:
Mosunetuzumab is a CD20xCD3 bispecific antibody that redirects T cells to engage and eliminate malignant B cells. Mosunetuzumab is an investigational agent in the United States.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal